Download high resolution image: pdf
Table 11: Rate constants for the reactions of CH3C(O)O2 with NO and NO2 at 760±20 Torr (N2 + O2 or air), unless otherwise stated.
| Reference | k(NO)a 298 K |
k(NO2)a 298 K |
k(NO)k(NO2) | Aa | E/R (K) | Temp range(K) | Method |
|---|---|---|---|---|---|---|---|
| Cox et al. (1976) | 1.7 | 298 | Relative | ||||
| Cox and Roffey (1977) | 1.8±0.4b | 294-328 | Relative | ||||
| Hendry and Kenley (1977) | 3.1b | 298-318 | Relative | ||||
| Kirchner et al. (1990) | 2.3±0.2b | 314-321 | Relative | ||||
| Tuazon et al. (1991) | 1.95±0.25b | 283-313 | Relative | ||||
| Seefeld et al. (1997) | 2.4±0.2b | 247-298 | Relative | ||||
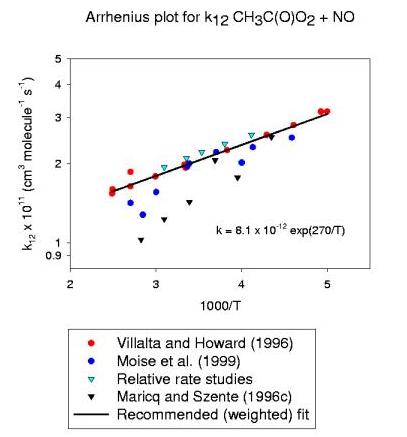
| Villalta and Howard (1996) | 2.0±0.3c | 0.81±0.13 | -270±60 | 200-402 | FT-MS | ||
| Maricq and Szente (1996) | 1.4±0.2d | 0.21 +1.4-0.8 | -570±140 | 230-354 | LP | ||
| Moise et al. (1999) | 1.8±0.3 | 0.60±0.11 | -320±40 | 218-370 | FT-MS | ||
| Sehested et al. (1998) | 2.0±0.3 | 1.0±0.2 | 2.07±0.21 | 298243-295 | PRRelative | ||
| Addison et al. (1980) | 0.47±0.03 | 298 | MMS | ||||
| Basco and Parmar (1987) | 0.48±0.05 | 298 | FP | ||||
| Bridier et al. (1991) | 0.96±0.09 | 0.21 | -450 | 248-393 | FP | ||
| Recommended | 2.0±0.3 | 1.0±0.2e | 2.0±0.3e | 0.81f 0.40e,g |
-270e,h |
a) Units of 10-11 cm3molecule-1s-1; b) Independent of temperature, within experimental uncertainties; c) Total pressure: 1-6 Torr (He); d) Total pressure: 100-120 Torr (N2+O2); e) At 760 Torr of N2 or air; f) For k(NO); g) For k(NO2); h) For both k(NO) and k(NO2), since their ratio has been shown to be temperature independent; the recommended value is from Bridier et al. FT-MS: Flow tube – mass spectrometry; LP: Laser-flash photolysis; PR: Pulse radiolysis; MMS: Molecular modulation spectrometry; FP: Flash photolysis.